Magnesium »
PDB 4pjk-4pyk »
4prx »
Magnesium in PDB 4prx: E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi
Enzymatic activity of E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi
All present enzymatic activity of E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi:
5.99.1.3;
5.99.1.3;
Protein crystallography data
The structure of E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi, PDB code: 4prx
was solved by
F.V.Stanger,
T.Schirmer,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.19 / 1.80 |
| Space group | C 2 2 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.640, 131.650, 92.390, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 16.3 / 20.3 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi
(pdb code 4prx). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi, PDB code: 4prx:
In total only one binding site of Magnesium was determined in the E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi, PDB code: 4prx:
Magnesium binding site 1 out of 1 in 4prx
Go back to
Magnesium binding site 1 out
of 1 in the E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi
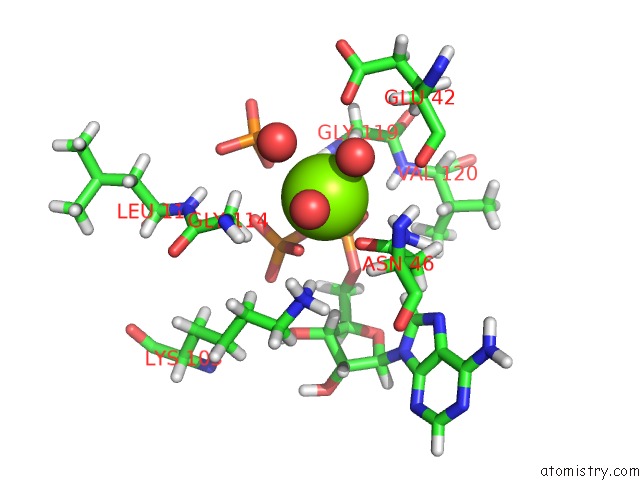
Mono view
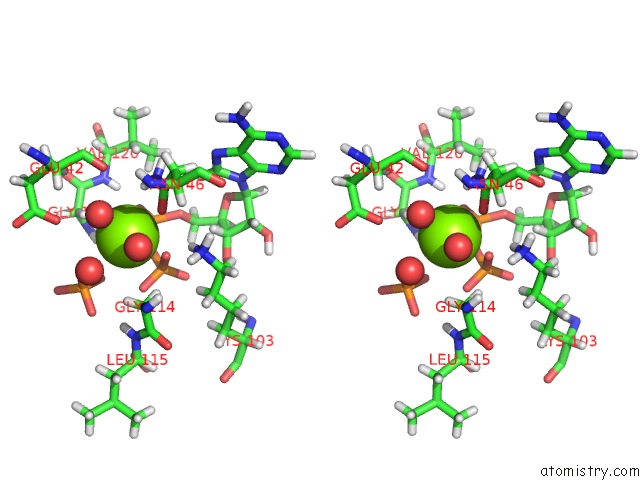
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of E. Coli Gyrb 43-kDa N-Terminal Fragment in Complex with Adp+Pi within 5.0Å range:
|
Reference:
F.V.Stanger,
C.Dehio,
T.Schirmer.
Structure of the N-Terminal Gyrase B Fragment in Complex with Adppi Reveals Rigid-Body Motion Induced By Atp Hydrolysis Plos One V. 9 07289 2014.
ISSN: ESSN 1932-6203
PubMed: 25202966
DOI: 10.1371/JOURNAL.PONE.0107289
Page generated: Mon Aug 11 22:08:32 2025
ISSN: ESSN 1932-6203
PubMed: 25202966
DOI: 10.1371/JOURNAL.PONE.0107289
Last articles
Mg in 5JC8Mg in 5JCA
Mg in 5JC7
Mg in 5JC3
Mg in 5JAC
Mg in 5JBH
Mg in 5JBQ
Mg in 5JBG
Mg in 5J9V
Mg in 5JB3