Magnesium »
PDB 4qq8-4qwi »
4qqf »
Magnesium in PDB 4qqf: Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
Protein crystallography data
The structure of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50, PDB code: 4qqf
was solved by
J.Z.Li,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 38.52 / 2.67 |
| Space group | P 41 21 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 164.287, 164.287, 150.532, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 21.2 / 25.2 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
(pdb code 4qqf). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50, PDB code: 4qqf:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50, PDB code: 4qqf:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
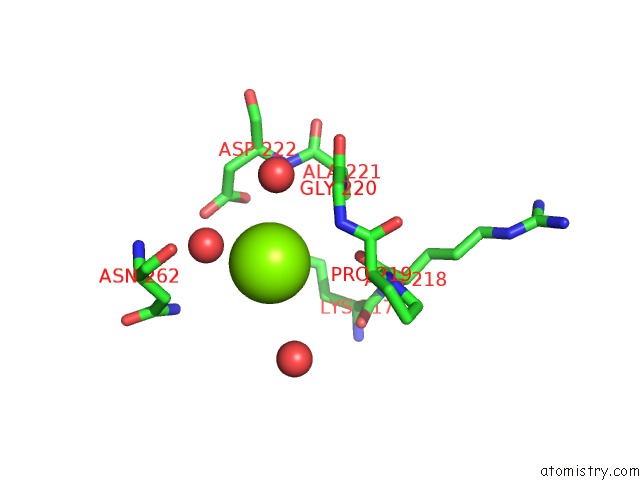
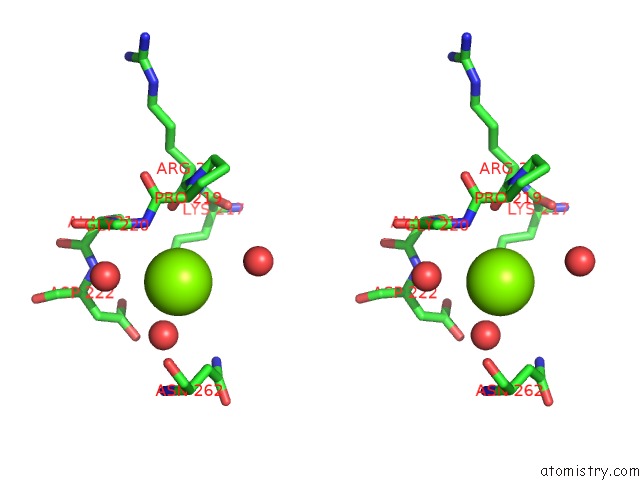
Magnesium binding site 1 out of 6 in 4qqf
Go back to
Magnesium binding site 1 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
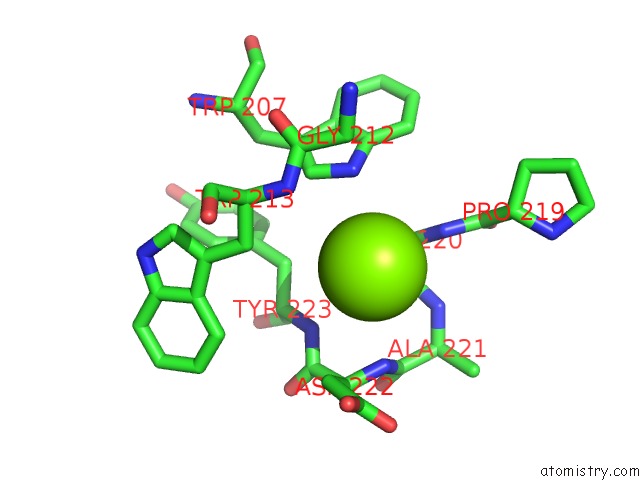
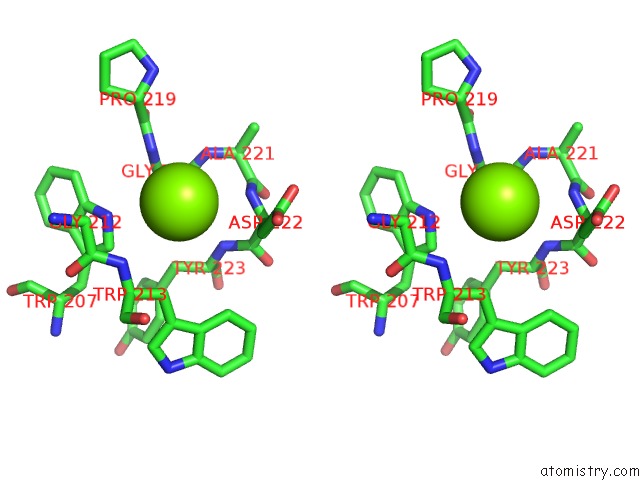
Magnesium binding site 2 out of 6 in 4qqf
Go back to
Magnesium binding site 2 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
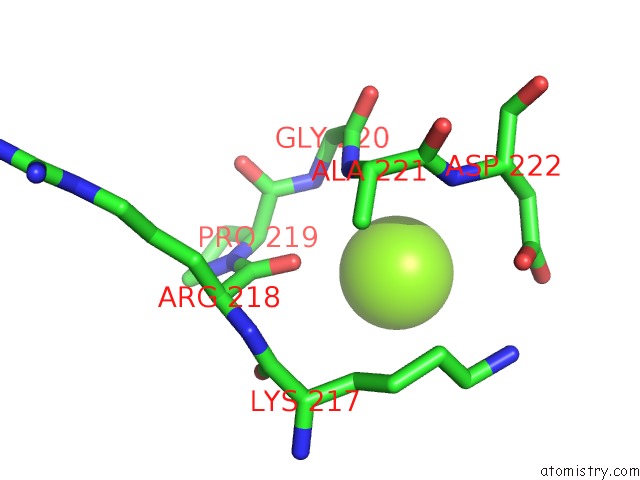
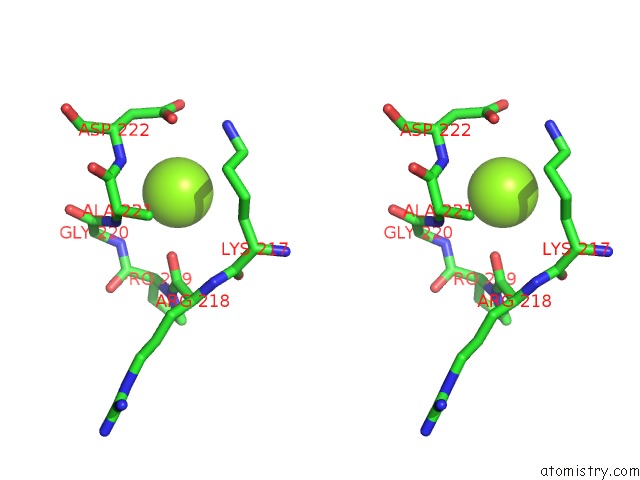
Magnesium binding site 3 out of 6 in 4qqf
Go back to
Magnesium binding site 3 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
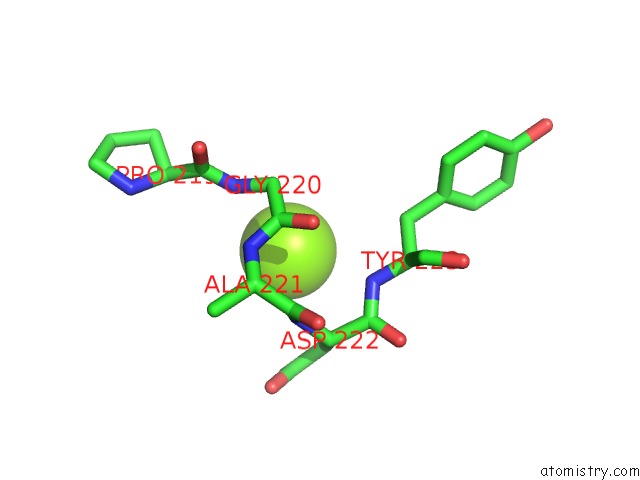
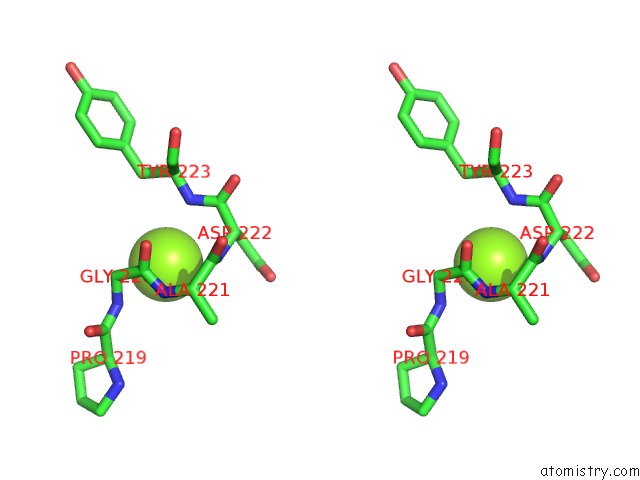
Magnesium binding site 4 out of 6 in 4qqf
Go back to
Magnesium binding site 4 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 4qqf
Go back to
Magnesium binding site 5 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
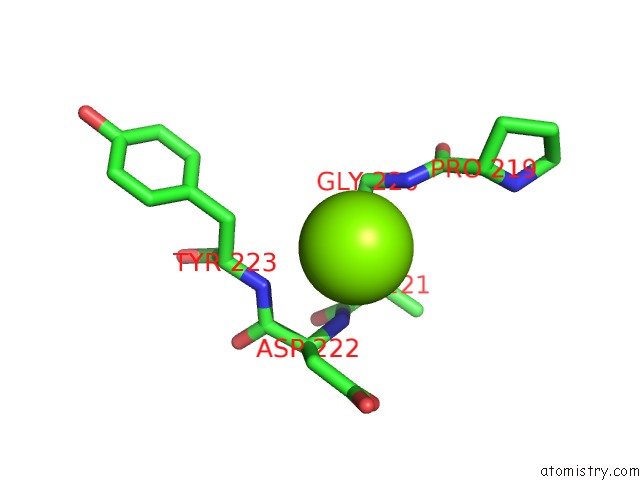
Mono view
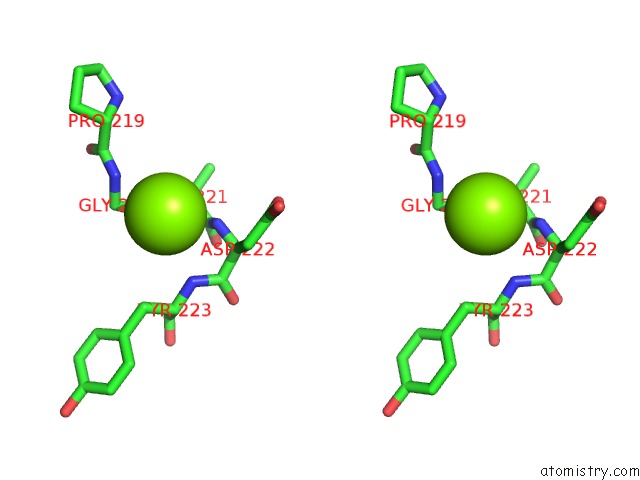
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 4qqf
Go back to
Magnesium binding site 6 out
of 6 in the Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50
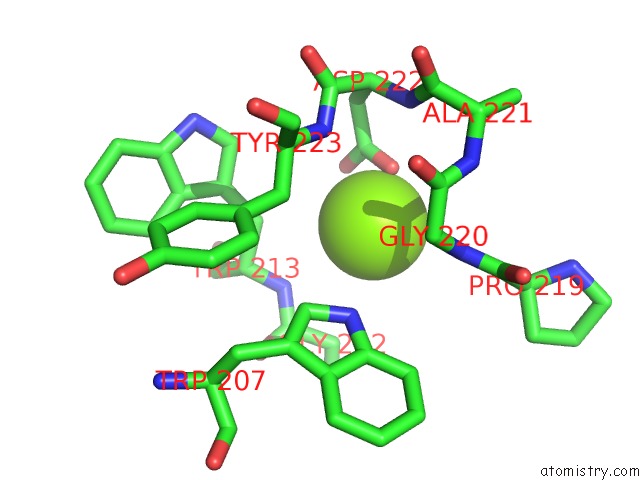
Mono view
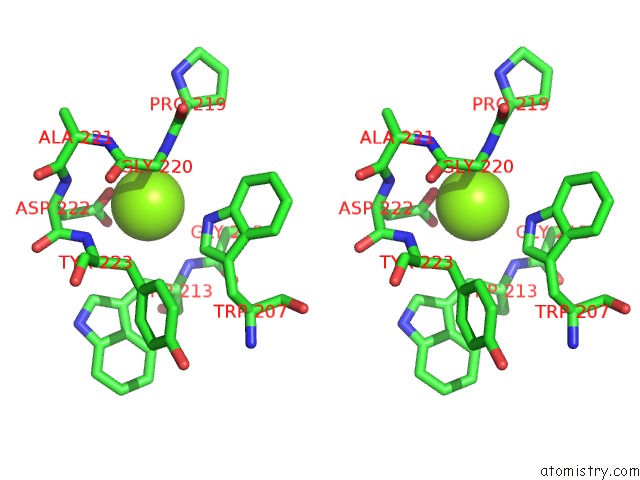
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Mitochondrial Import Inner Membrane Translocase Subunit TIM50 within 5.0Å range:
|
Reference:
J.Li,
B.Sha.
The Structure of TIM50(164-361) Suggests the Mechanism By Which TIM50 Receives Mitochondrial Presequences. Acta Crystallogr F Struct V. 71 1146 2015BIOL Commun.
ISSN: ESSN 2053-230X
PubMed: 26323300
DOI: 10.1107/S2053230X15013102
Page generated: Mon Aug 11 22:33:04 2025
ISSN: ESSN 2053-230X
PubMed: 26323300
DOI: 10.1107/S2053230X15013102
Last articles
Mg in 5IY6Mg in 5IY5
Mg in 5IWY
Mg in 5IXT
Mg in 5IWX
Mg in 5IXQ
Mg in 5IXO
Mg in 5IX1
Mg in 5IX2
Mg in 5IWA