Magnesium »
PDB 6bd1-6br7 »
6bfz »
Magnesium in PDB 6bfz: Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
Enzymatic activity of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
All present enzymatic activity of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form:
4.2.1.11;
4.2.1.11;
Protein crystallography data
The structure of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form, PDB code: 6bfz
was solved by
H.Erlandsen,
D.Wright,
J.Krucinska,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.69 / 2.21 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 111.262, 143.293, 207.040, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.1 / 21.8 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
(pdb code 6bfz). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form, PDB code: 6bfz:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
In total 6 binding sites of Magnesium where determined in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form, PDB code: 6bfz:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6;
Magnesium binding site 1 out of 6 in 6bfz
Go back to
Magnesium binding site 1 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
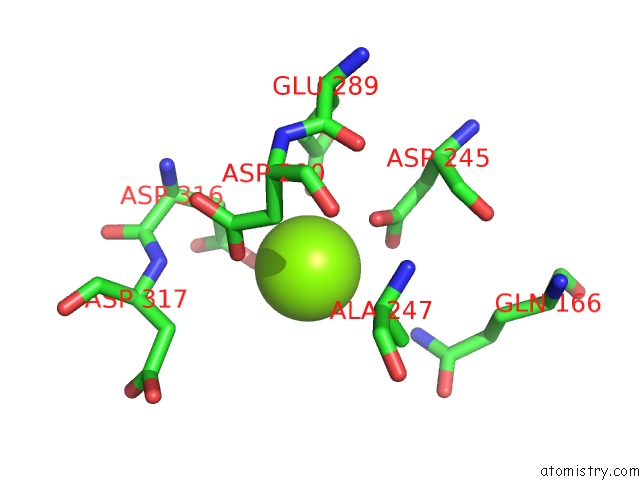
Mono view
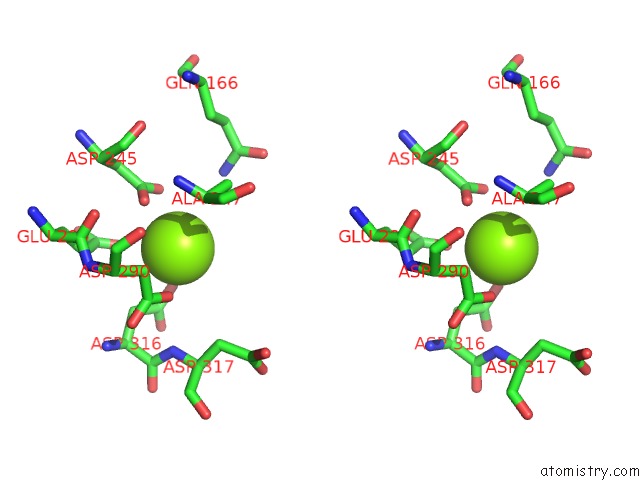
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Magnesium binding site 2 out of 6 in 6bfz
Go back to
Magnesium binding site 2 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
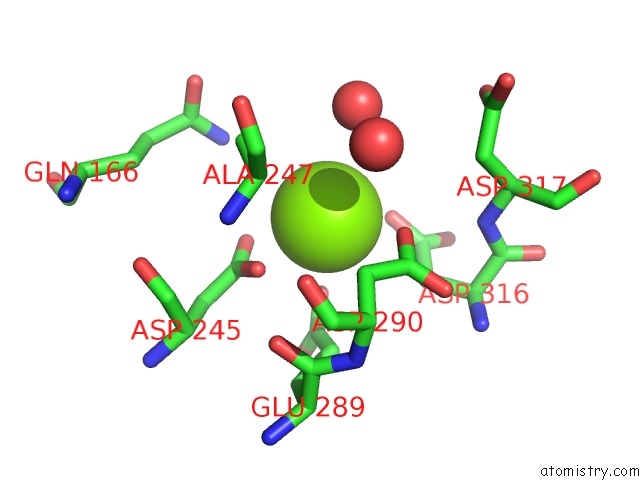
Mono view
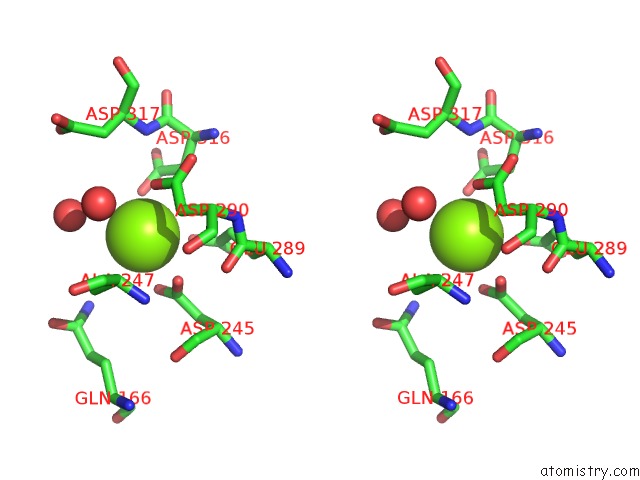
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Magnesium binding site 3 out of 6 in 6bfz
Go back to
Magnesium binding site 3 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
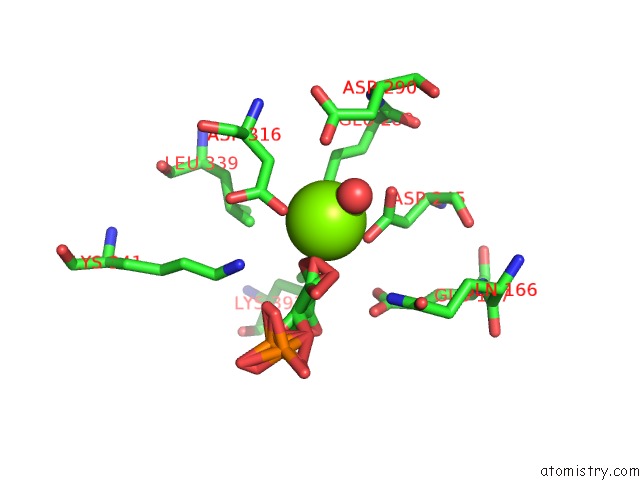
Mono view
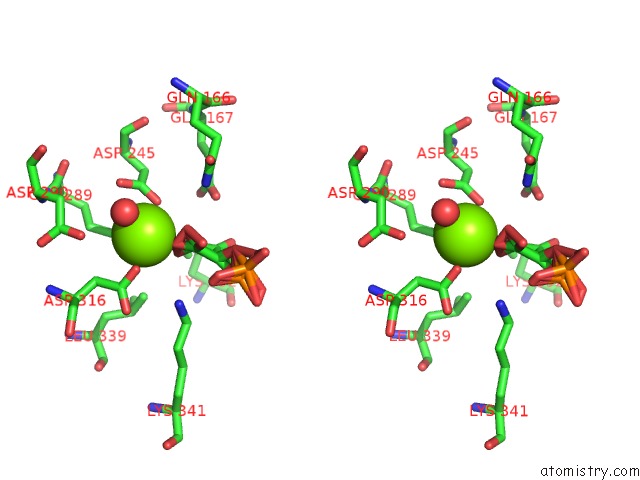
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Magnesium binding site 4 out of 6 in 6bfz
Go back to
Magnesium binding site 4 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
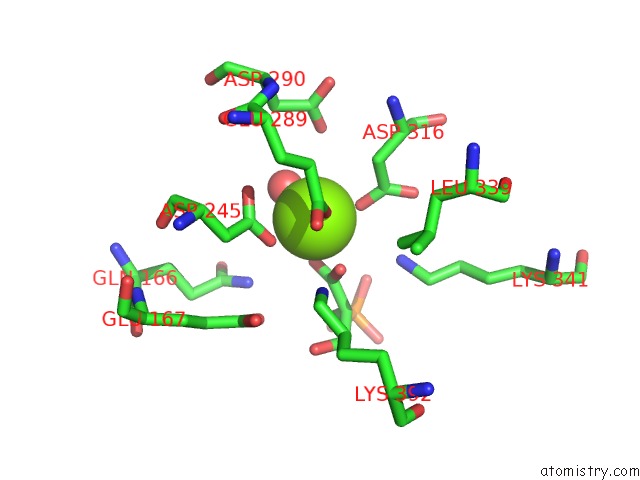
Mono view
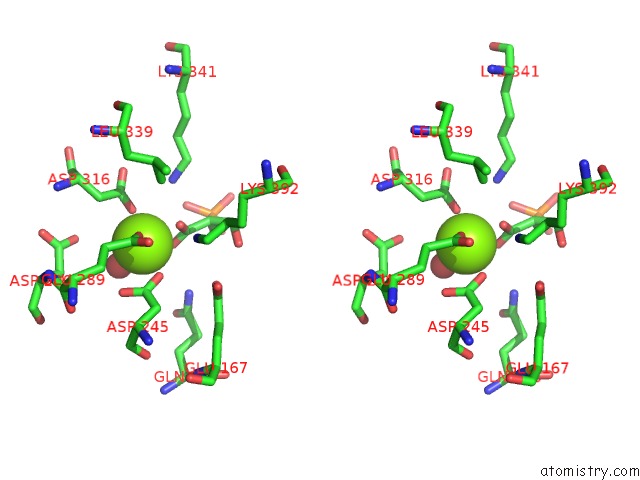
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Magnesium binding site 5 out of 6 in 6bfz
Go back to
Magnesium binding site 5 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
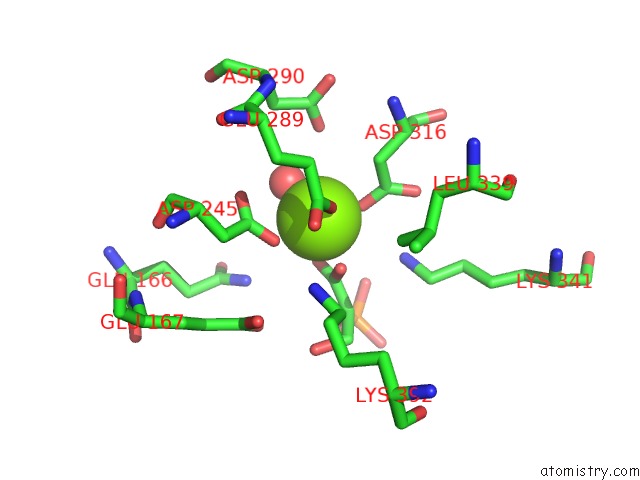
Mono view
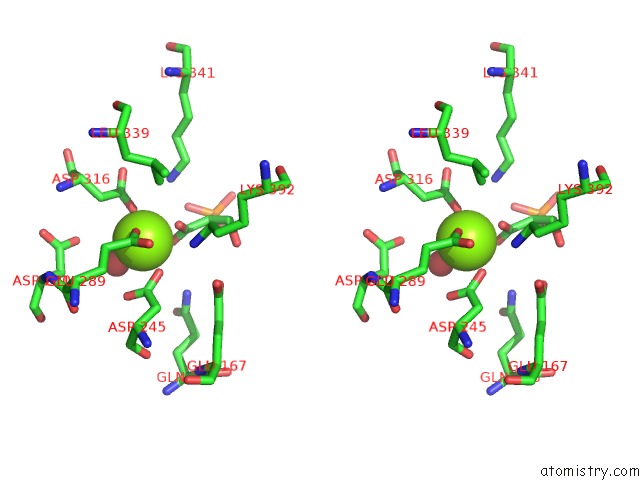
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Magnesium binding site 6 out of 6 in 6bfz
Go back to
Magnesium binding site 6 out
of 6 in the Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form
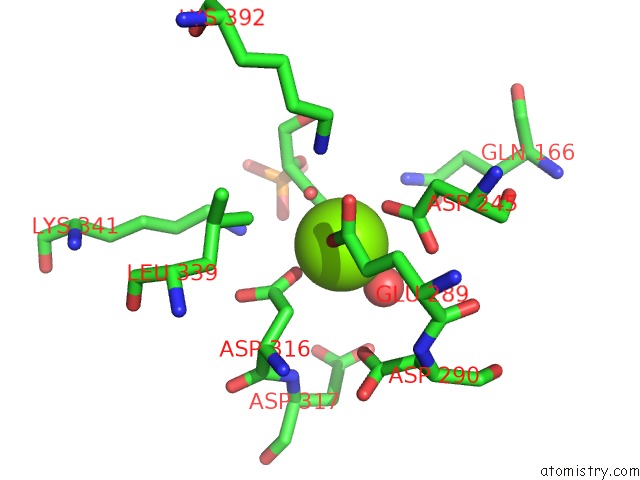
Mono view
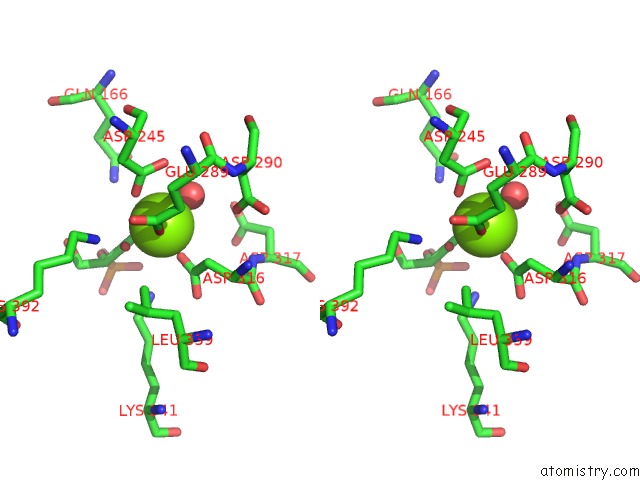
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of Enolase From E. Coli with A Mixture of Apo Form, Substrate, and Product Form within 5.0Å range:
|
Reference:
J.Krucinska,
E.Falcone,
H.Erlandsen,
A.Hazeen,
M.N.Lombardo,
A.Estrada,
V.L.Robinson,
A.C.Anderson,
D.L.Wright.
Structural and Functional Studies of Bacterial Enolase, A Potential Target Against Gram-Negative Pathogens. Biochemistry V. 58 1188 2019.
ISSN: ISSN 1520-4995
PubMed: 30714720
DOI: 10.1021/ACS.BIOCHEM.8B01298
Page generated: Wed Aug 13 02:37:57 2025
ISSN: ISSN 1520-4995
PubMed: 30714720
DOI: 10.1021/ACS.BIOCHEM.8B01298
Last articles
Mg in 6VGMMg in 6VJJ
Mg in 6VIK
Mg in 6VIJ
Mg in 6VII
Mg in 6VFX
Mg in 6VFS
Mg in 6VGG
Mg in 6VEC
Mg in 6VG6