Magnesium »
PDB 6ll8-6ly7 »
6lla »
Magnesium in PDB 6lla: Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
Enzymatic activity of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
All present enzymatic activity of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad:
4.2.3.4;
4.2.3.4;
Protein crystallography data
The structure of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad, PDB code: 6lla
was solved by
N.Neetu,
M.Katiki,
P.Kumar,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 143.46 / 1.88 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 89.390, 59.920, 143.800, 90.00, 93.95, 90.00 |
| R / Rfree (%) | 17.3 / 23.8 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
(pdb code 6lla). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad, PDB code: 6lla:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad, PDB code: 6lla:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 6lla
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
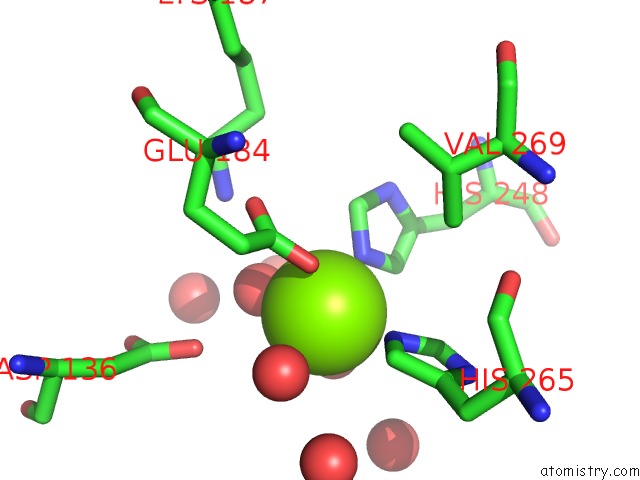
Mono view
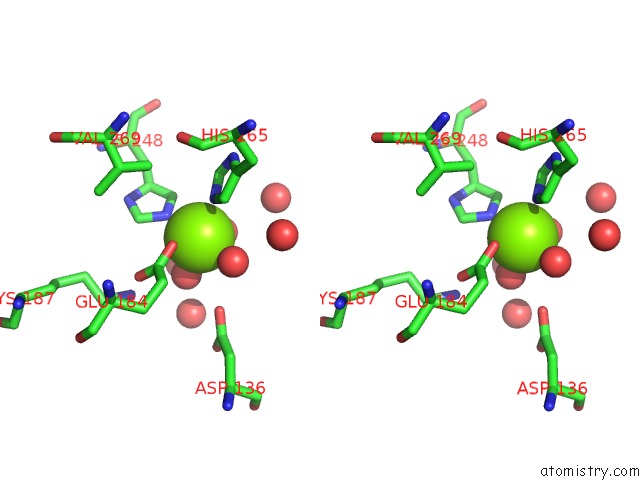
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 6lla
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
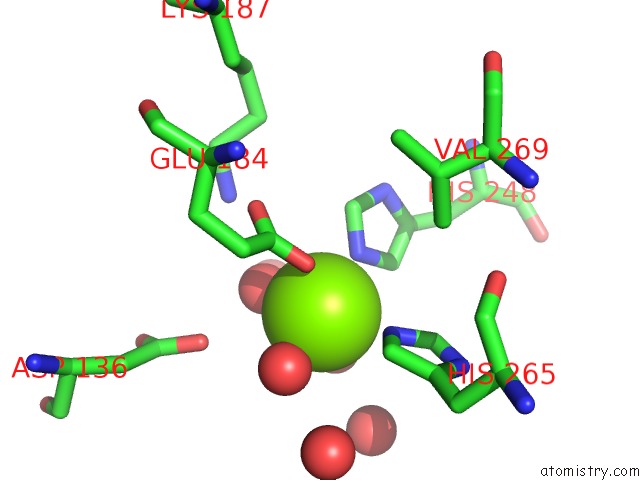
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 6lla
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
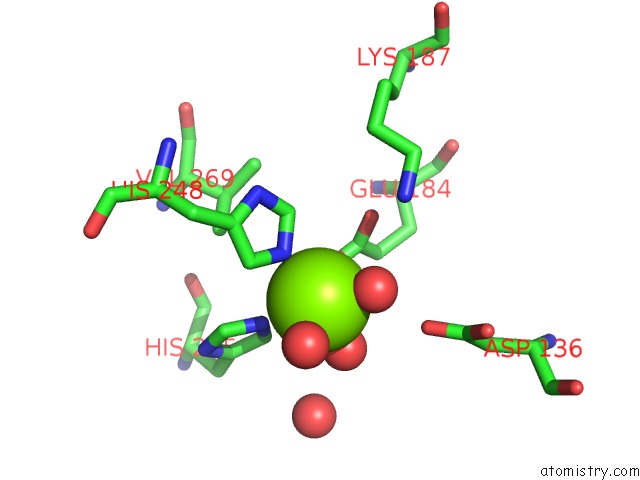
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 6lla
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad
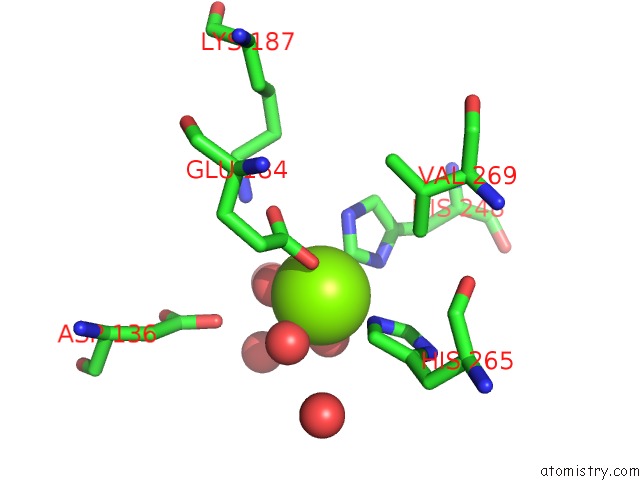
Mono view
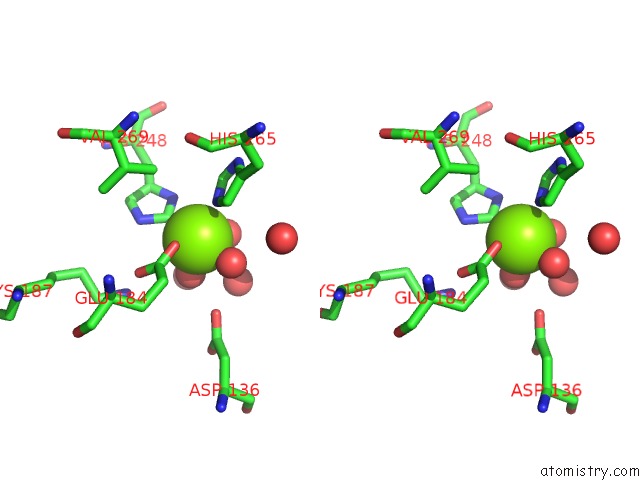
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Providencia Alcalifaciens 3-Dehydroquinate Synthase (Dhqs) in Complex with MG2+ and Nad within 5.0Å range:
|
Reference:
N.Neetu,
M.Katiki,
A.Dev,
S.Gaur,
S.Tomar,
P.Kumar.
Structural and Biochemical Analyses Reveal That Chlorogenic Acid Inhibits the Shikimate Pathway. J.Bacteriol. V. 202 2020.
ISSN: ESSN 1098-5530
PubMed: 32661075
DOI: 10.1128/JB.00248-20
Page generated: Wed Aug 13 11:24:06 2025
ISSN: ESSN 1098-5530
PubMed: 32661075
DOI: 10.1128/JB.00248-20
Last articles
Mg in 6O3PMg in 6O2R
Mg in 6O2Q
Mg in 6O36
Mg in 6O2P
Mg in 6O1V
Mg in 6O0J
Mg in 6O1E
Mg in 6O0G
Mg in 6NYY