Magnesium »
PDB 6ll8-6ly7 »
6ltw »
Magnesium in PDB 6ltw: Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
Enzymatic activity of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
All present enzymatic activity of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis:
2.5.1.6;
2.5.1.6;
Protein crystallography data
The structure of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis, PDB code: 6ltw
was solved by
R.K.Singh,
F.Michailidou,
A.Rentmeister,
D.Kuemmel,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 48.08 / 1.65 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 62.877, 100.360, 66.842, 90.00, 95.86, 90.00 |
| R / Rfree (%) | 15.6 / 19.1 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
(pdb code 6ltw). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis, PDB code: 6ltw:
Jump to Magnesium binding site number: 1; 2; 3; 4;
In total 4 binding sites of Magnesium where determined in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis, PDB code: 6ltw:
Jump to Magnesium binding site number: 1; 2; 3; 4;
Magnesium binding site 1 out of 4 in 6ltw
Go back to
Magnesium binding site 1 out
of 4 in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
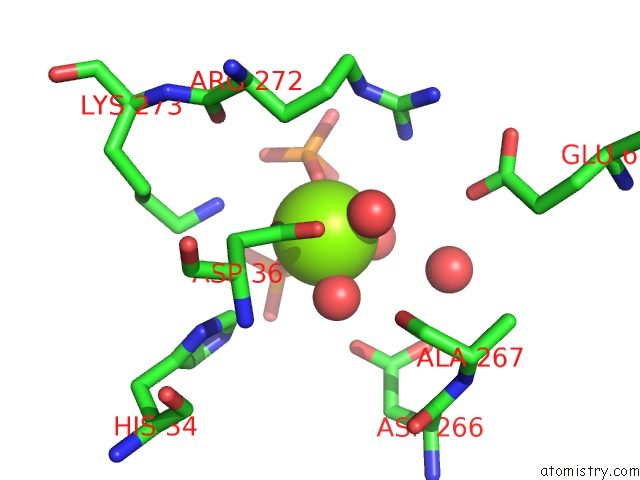
Mono view
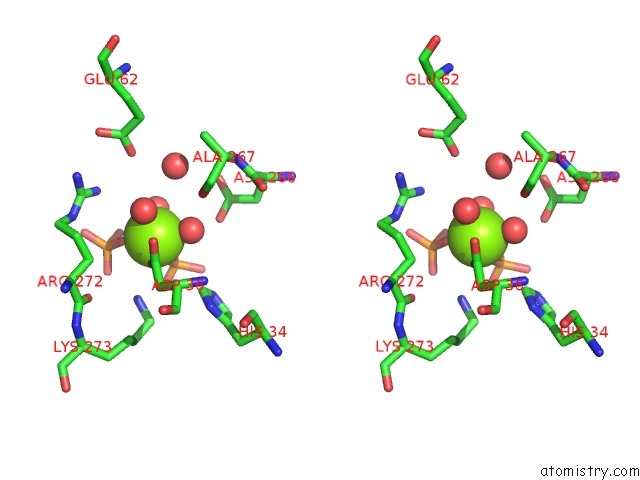
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis within 5.0Å range:
|
Magnesium binding site 2 out of 4 in 6ltw
Go back to
Magnesium binding site 2 out
of 4 in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
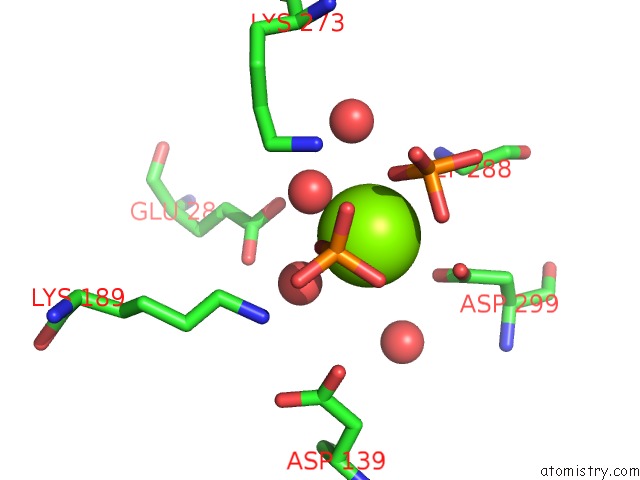
Mono view
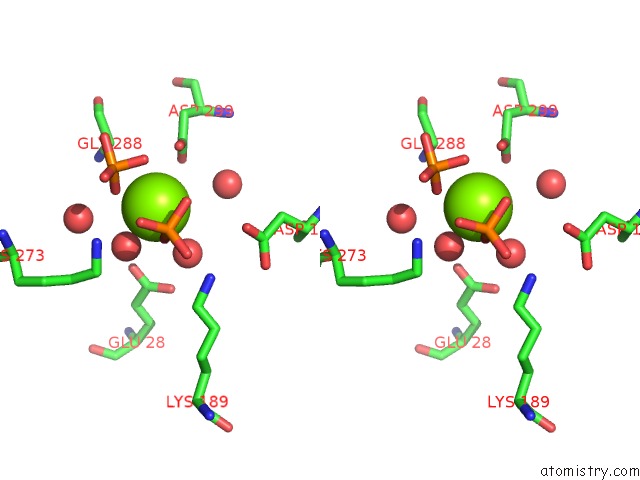
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis within 5.0Å range:
|
Magnesium binding site 3 out of 4 in 6ltw
Go back to
Magnesium binding site 3 out
of 4 in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
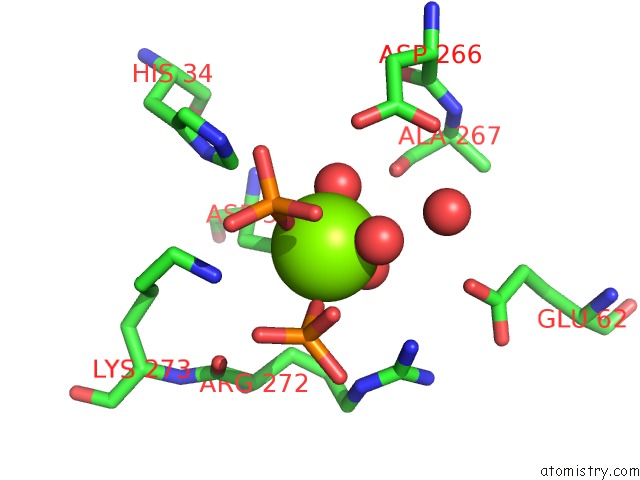
Mono view
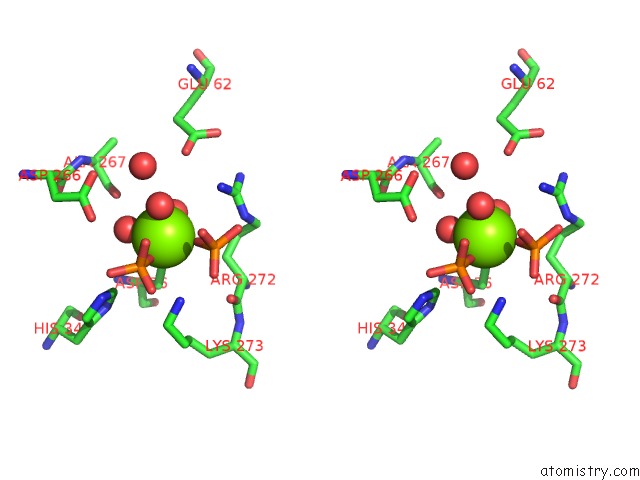
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis within 5.0Å range:
|
Magnesium binding site 4 out of 4 in 6ltw
Go back to
Magnesium binding site 4 out
of 4 in the Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis
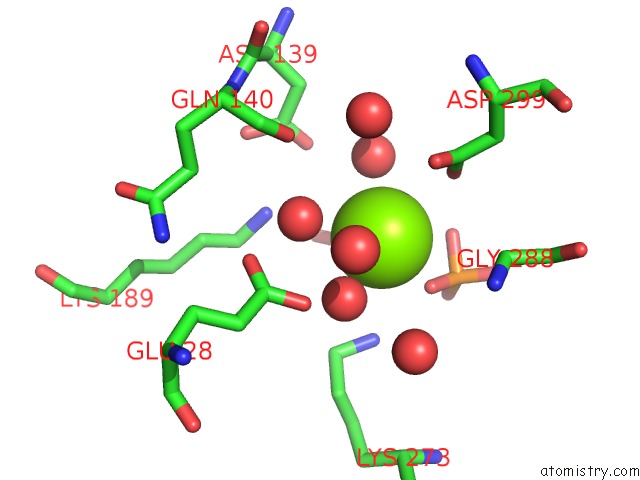
Mono view
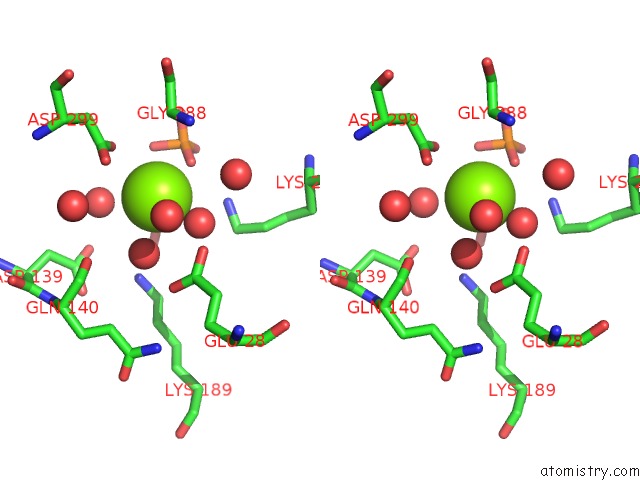
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of Apo Form of I122A/I330A Variant of S- Adenosylmethionine Synthetase From Cryptosporidium Hominis within 5.0Å range:
|
Reference:
F.Michailidou,
N.Klocker,
N.Cornelissen,
R.K.Singh,
A.Peters,
A.Ovcharenko,
D.Kummel,
A.Rentmeister.
Engineered Sam Synthetases For Enzymatic Generation of Adomet Analogs with Photocaging Groups and Reversible Dna Modification in Cascade Reactions. Angew.Chem.Int.Ed.Engl. 2020.
ISSN: ESSN 1521-3773
PubMed: 33017502
DOI: 10.1002/ANIE.202012623
Page generated: Wed Aug 13 11:29:17 2025
ISSN: ESSN 1521-3773
PubMed: 33017502
DOI: 10.1002/ANIE.202012623
Last articles
Mg in 7EWAMg in 7EW9
Mg in 7EV7
Mg in 7EVI
Mg in 7EUQ
Mg in 7EUN
Mg in 7EV3
Mg in 7EV2
Mg in 7EUL
Mg in 7EUK