Magnesium »
PDB 8e1u-8ec1 »
8e8u »
Magnesium in PDB 8e8u: Structure of the Lor Domain of Human Aass
Enzymatic activity of Structure of the Lor Domain of Human Aass
Protein crystallography data
The structure of Structure of the Lor Domain of Human Aass, PDB code: 8e8u
was solved by
S.Khamrui,
M.B.Lazarus,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.41 / 2.65 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 73.005, 131.944, 96.494, 90, 100.72, 90 |
| R / Rfree (%) | 27.8 / 31.9 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Structure of the Lor Domain of Human Aass
(pdb code 8e8u). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Structure of the Lor Domain of Human Aass, PDB code: 8e8u:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Structure of the Lor Domain of Human Aass, PDB code: 8e8u:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 8e8u
Go back to
Magnesium binding site 1 out
of 2 in the Structure of the Lor Domain of Human Aass
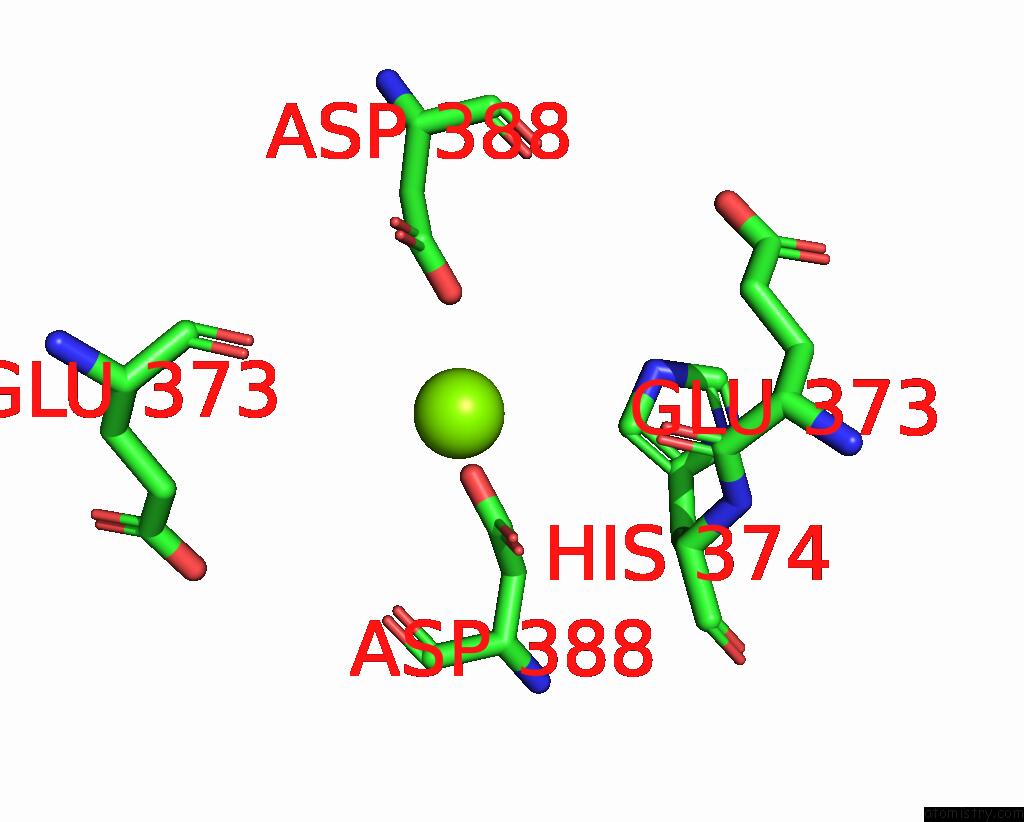
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Structure of the Lor Domain of Human Aass within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 8e8u
Go back to
Magnesium binding site 2 out
of 2 in the Structure of the Lor Domain of Human Aass
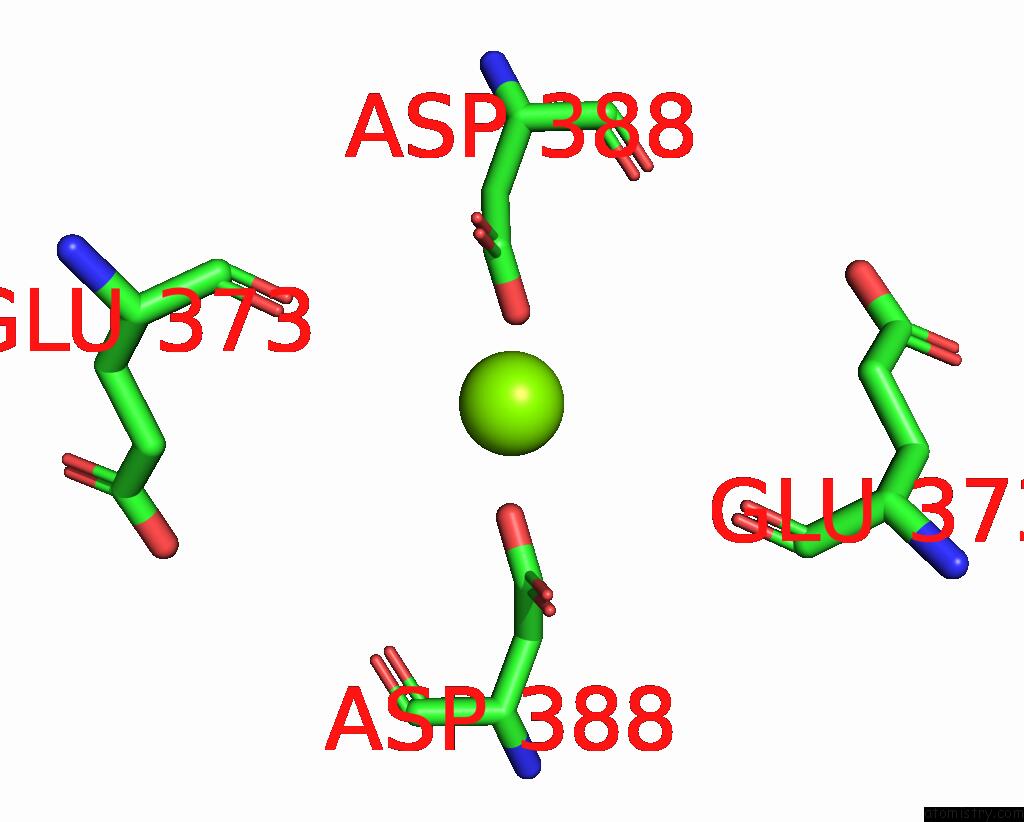
Mono view
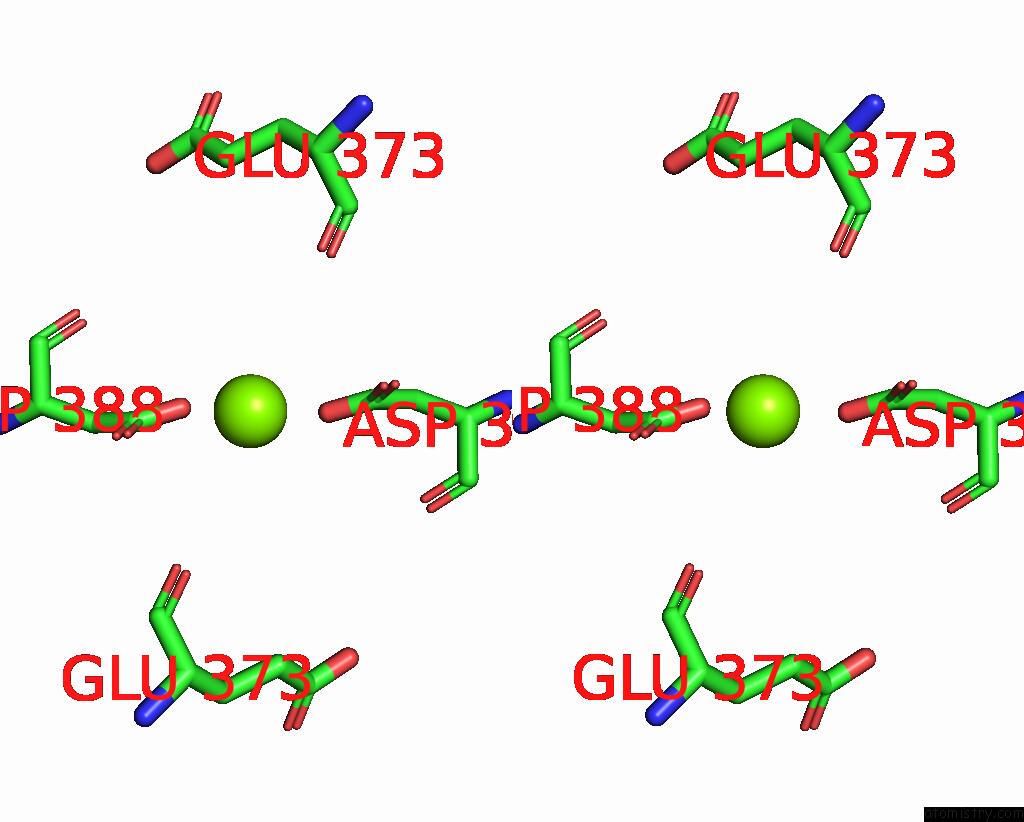
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Structure of the Lor Domain of Human Aass within 5.0Å range:
|
Reference:
J.Leandro,
S.Khamrui,
C.Suebsuwong,
P.J.Chen,
C.Secor,
T.Dodatko,
C.Yu,
R.Sanchez,
R.J.Devita,
S.M.Houten,
M.B.Lazarus.
Characterization and Structure of the Human Lysine-2-Oxoglutarate Reductase Domain, A Novel Therapeutic Target For Treatment of Glutaric Aciduria Type 1. Open Biology V. 12 20179 2022.
ISSN: ESSN 2046-2441
PubMed: 36128717
DOI: 10.1098/RSOB.220179
Page generated: Fri Aug 15 03:40:32 2025
ISSN: ESSN 2046-2441
PubMed: 36128717
DOI: 10.1098/RSOB.220179
Last articles
Mg in 8IWUMg in 8IWT
Mg in 8IWS
Mg in 8IWR
Mg in 8IWN
Mg in 8IU7
Mg in 8IUH
Mg in 8IUE
Mg in 8ITS
Mg in 8ITY