Magnesium »
PDB 8t7v-8teb »
8t7v »
Magnesium in PDB 8t7v: Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde)
Enzymatic activity of Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde)
All present enzymatic activity of Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde):
3.6.5.2;
3.6.5.2;
Protein crystallography data
The structure of Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde), PDB code: 8t7v
was solved by
J.G.H.Bruystens,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 46.24 / 2.25 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 57.546, 77.805, 58.827, 90, 92.19, 90 |
| R / Rfree (%) | 20.2 / 25 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde)
(pdb code 8t7v). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde), PDB code: 8t7v:
In total only one binding site of Magnesium was determined in the Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde), PDB code: 8t7v:
Magnesium binding site 1 out of 1 in 8t7v
Go back to
Magnesium binding site 1 out
of 1 in the Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde)

Mono view
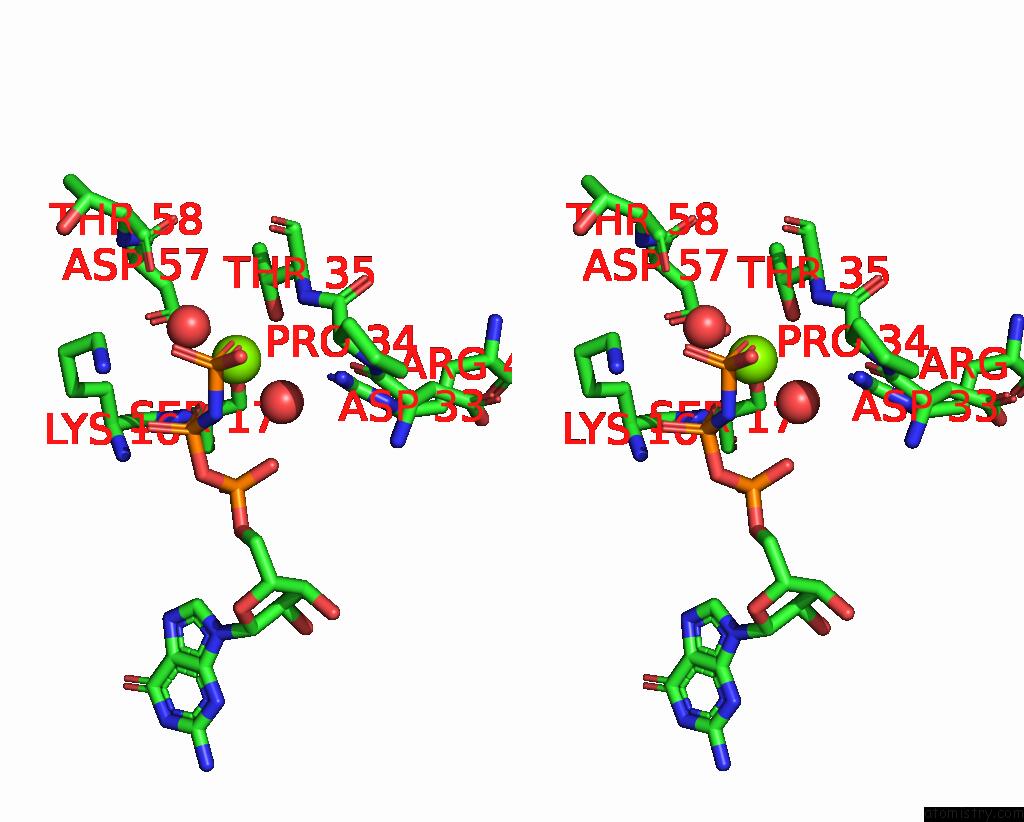
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Co-Crystal Structure of KRIT1 with A 1-Hydroxy 2-Naphthaldehyde Derivative (6-(Furan-2-Yl)-2-Hydroxy-1-Naphthaldehyde) within 5.0Å range:
|
Reference:
K.R.Francisco,
J.Bruystens,
C.Varricchio,
S.Mccurdy,
J.Wu,
M.A.Lopez-Ramirez,
M.Ginsberg,
C.R.Caffrey,
A.Brancale,
A.R.Gingras,
M.S.Hixon,
C.Ballatore.
Targeted Reversible Covalent Modification of A Noncatalytic Lysine of the Krev Interaction Trapped 1 Protein Enables Site-Directed Screening For Protein-Protein Interaction Inhibitors. Acs Pharmacol Transl Sci V. 6 1651 2023.
ISSN: ESSN 2575-910
PubMed: 37974623
DOI: 10.1021/ACSPTSCI.3C00156
Page generated: Fri Aug 15 16:16:57 2025
ISSN: ESSN 2575-910
PubMed: 37974623
DOI: 10.1021/ACSPTSCI.3C00156
Last articles
Mg in 8WKGMg in 8WKF
Mg in 8WIM
Mg in 8WIL
Mg in 8WH2
Mg in 8WH0
Mg in 8WGY
Mg in 8WDV
Mg in 8WGO
Mg in 8WDU