Magnesium »
PDB 4qhe-4qpz »
4qjb »
Magnesium in PDB 4qjb: Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum
Protein crystallography data
The structure of Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum, PDB code: 4qjb
was solved by
N.H.Tolia,
J.Park,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.46 / 2.05 |
| Space group | P 1 21 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 77.900, 43.800, 83.900, 90.00, 101.10, 90.00 |
| R / Rfree (%) | 20.2 / 24.2 |
Other elements in 4qjb:
The structure of Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum also contains other interesting chemical elements:
| Chlorine | (Cl) | 2 atoms |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum
(pdb code 4qjb). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 2 binding sites of Magnesium where determined in the Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum, PDB code: 4qjb:
Jump to Magnesium binding site number: 1; 2;
In total 2 binding sites of Magnesium where determined in the Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum, PDB code: 4qjb:
Jump to Magnesium binding site number: 1; 2;
Magnesium binding site 1 out of 2 in 4qjb
Go back to
Magnesium binding site 1 out
of 2 in the Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum

Mono view
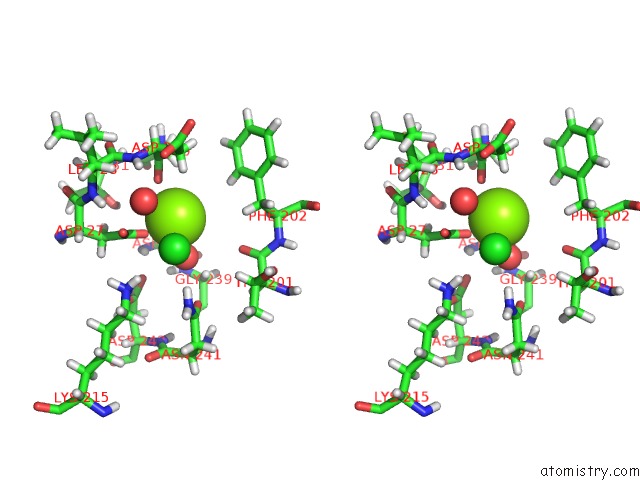
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum within 5.0Å range:
|
Magnesium binding site 2 out of 2 in 4qjb
Go back to
Magnesium binding site 2 out
of 2 in the Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum
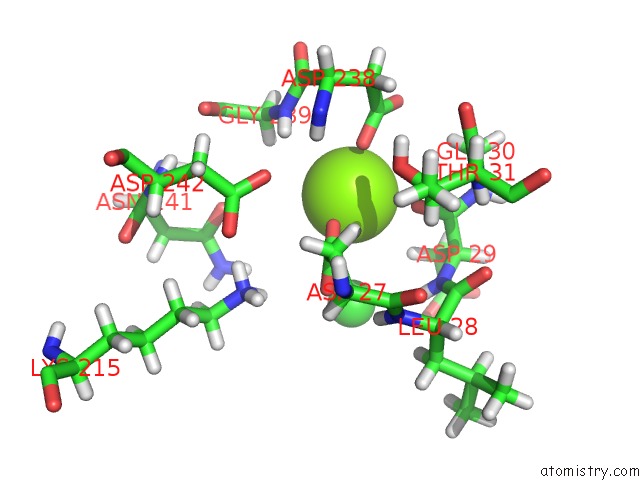
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of the Sugar Phosphatase PFHAD1 From Plasmodium Falciparum within 5.0Å range:
|
Reference:
A.M.Guggisberg,
J.Park,
R.L.Edwards,
M.L.Kelly,
D.M.Hodge,
N.H.Tolia,
A.R.Odom.
A Sugar Phosphatase Regulates the Methylerythritol Phosphate (Mep) Pathway in Malaria Parasites. Nat Commun V. 5 4467 2014.
ISSN: ESSN 2041-1723
PubMed: 25058848
DOI: 10.1038/NCOMMS5467
Page generated: Mon Aug 11 22:26:18 2025
ISSN: ESSN 2041-1723
PubMed: 25058848
DOI: 10.1038/NCOMMS5467
Last articles
Mg in 5FTNMg in 5FUX
Mg in 5FUK
Mg in 5FTM
Mg in 5FUJ
Mg in 5FUI
Mg in 5FTE
Mg in 5FTB
Mg in 5FSC
Mg in 5FSB