Magnesium »
PDB 6fkf-6ftv »
6fm2 »
Magnesium in PDB 6fm2: Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin
Protein crystallography data
The structure of Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin, PDB code: 6fm2
was solved by
T.M.Kotila,
K.Kogan,
P.Lappalainen,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 39.57 / 2.80 |
| Space group | P 61 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 73.833, 73.833, 453.377, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 18.3 / 23.4 |
Magnesium Binding Sites:
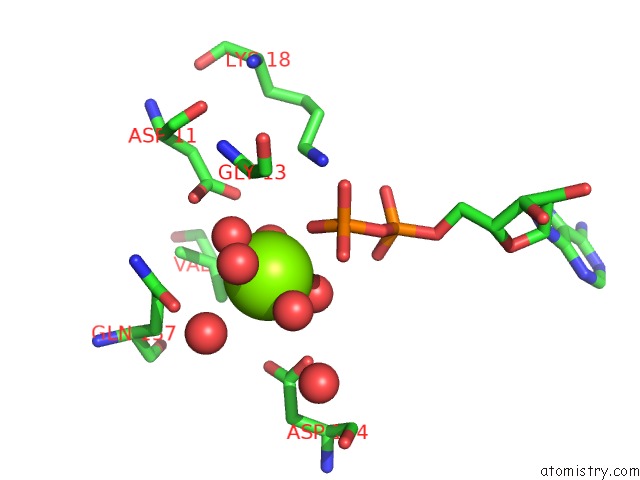
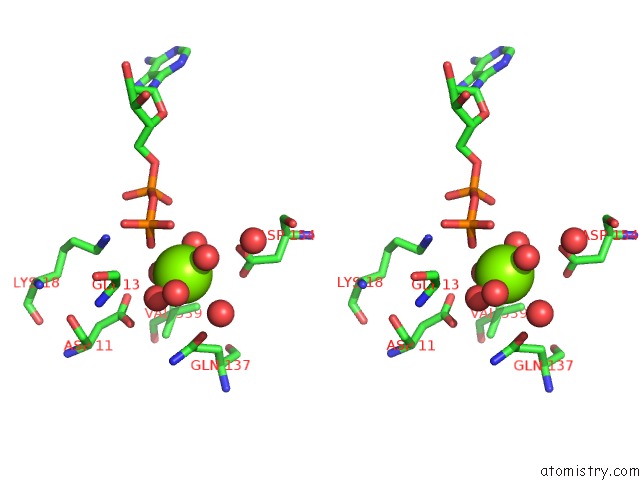
The binding sites of Magnesium atom in the Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin
(pdb code 6fm2). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total only one binding site of Magnesium was determined in the Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin, PDB code: 6fm2:
In total only one binding site of Magnesium was determined in the Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin, PDB code: 6fm2:
Magnesium binding site 1 out of 1 in 6fm2
Go back to
Magnesium binding site 1 out
of 1 in the Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Carp Domain of Mouse Cyclase-Associated Protein 1 (CAP1) Bound to Adp- Actin within 5.0Å range:
|
Reference:
T.Kotila,
K.Kogan,
G.Enkavi,
S.Guo,
I.Vattulainen,
B.L.Goode,
P.Lappalainen.
Structural Basis of Actin Monomer Re-Charging By Cyclase-Associated Protein. Nat Commun V. 9 1892 2018.
ISSN: ESSN 2041-1723
PubMed: 29760438
DOI: 10.1038/S41467-018-04231-7
Page generated: Wed Aug 13 06:09:24 2025
ISSN: ESSN 2041-1723
PubMed: 29760438
DOI: 10.1038/S41467-018-04231-7
Last articles
Mg in 7CVHMg in 7CTX
Mg in 7CU9
Mg in 7CQU
Mg in 7CQQ
Mg in 7CTV
Mg in 7CTT
Mg in 7CT3
Mg in 7CPQ
Mg in 7CPD