Magnesium »
PDB 5ets-5f7u »
5f49 »
Magnesium in PDB 5f49: Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
Protein crystallography data
The structure of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A, PDB code: 5f49
was solved by
Z.Xu,
T.Skarina,
P.J.Stogios,
V.Yim,
A.Savchenko,
W.F.Anderson,
Center Forstructural Genomics Of Infectious Diseases (Csgid),
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 23.49 / 2.15 |
| Space group | P 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 46.159, 54.047, 84.847, 72.30, 74.57, 88.07 |
| R / Rfree (%) | 19.2 / 22.7 |
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
(pdb code 5f49). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 8 binding sites of Magnesium where determined in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A, PDB code: 5f49:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
In total 8 binding sites of Magnesium where determined in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A, PDB code: 5f49:
Jump to Magnesium binding site number: 1; 2; 3; 4; 5; 6; 7; 8;
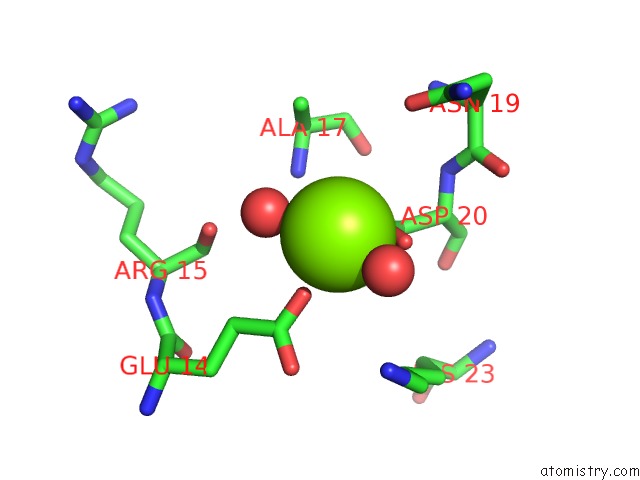
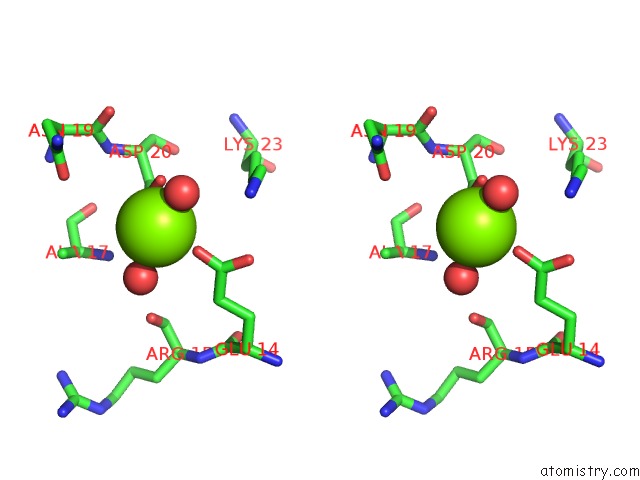
Magnesium binding site 1 out of 8 in 5f49
Go back to
Magnesium binding site 1 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
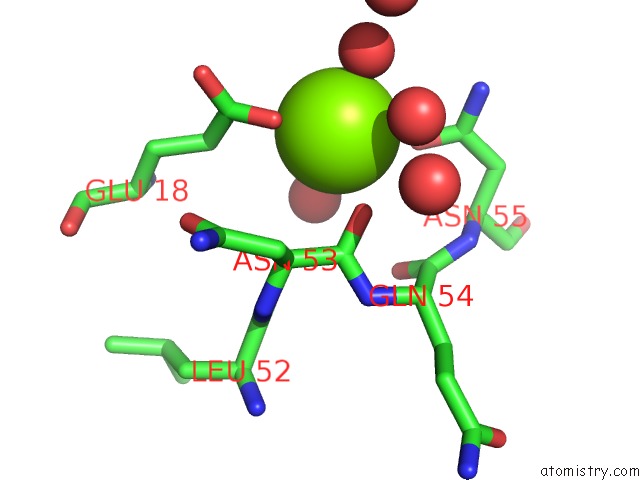
Mono view
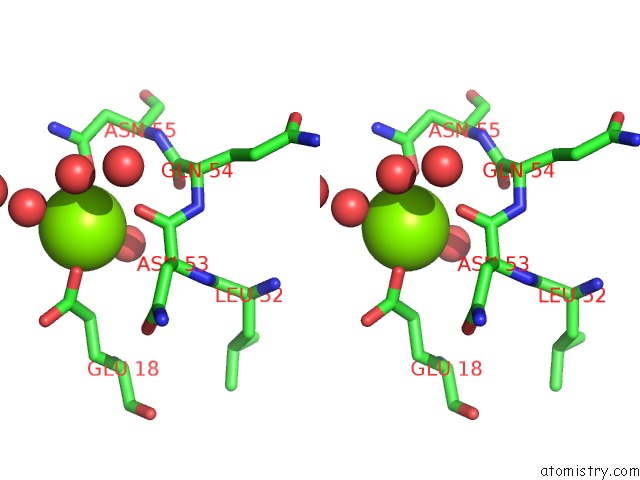
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Magnesium binding site 2 out of 8 in 5f49
Go back to
Magnesium binding site 2 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
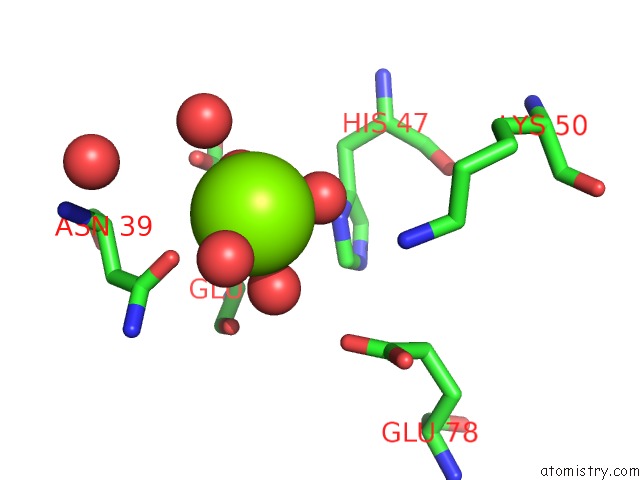
Mono view
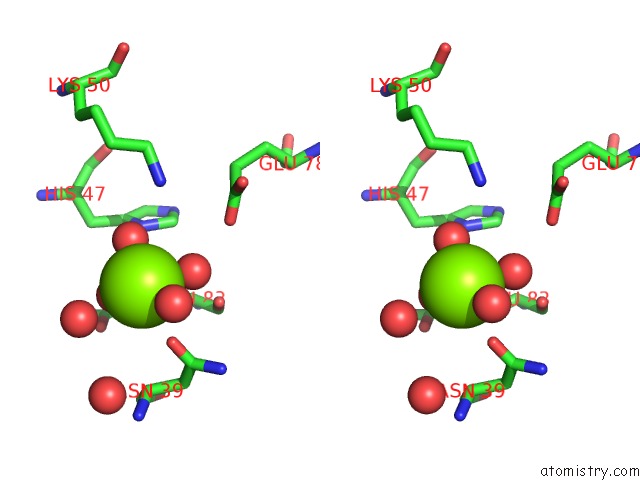
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Magnesium binding site 3 out of 8 in 5f49
Go back to
Magnesium binding site 3 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Magnesium binding site 4 out of 8 in 5f49
Go back to
Magnesium binding site 4 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 4 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Magnesium binding site 5 out of 8 in 5f49
Go back to
Magnesium binding site 5 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
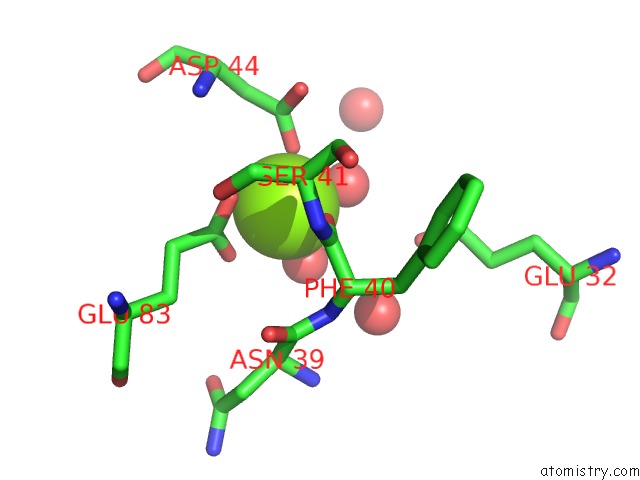
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 5 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Magnesium binding site 6 out of 8 in 5f49
Go back to
Magnesium binding site 6 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
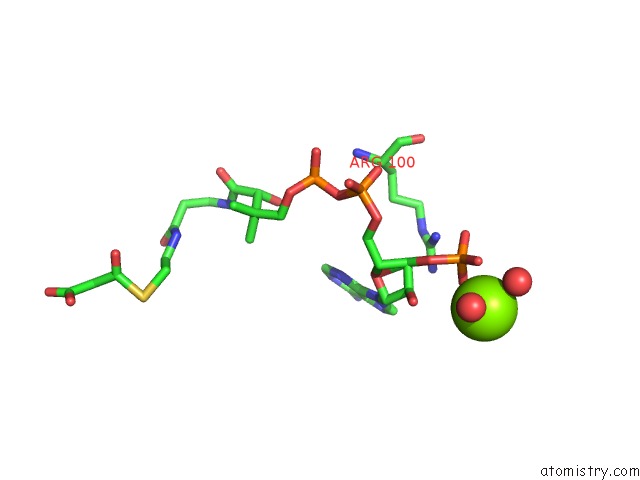
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 6 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
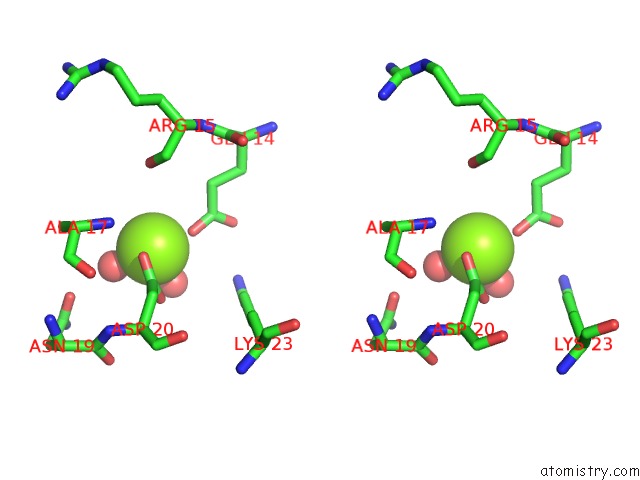
Magnesium binding site 7 out of 8 in 5f49
Go back to
Magnesium binding site 7 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
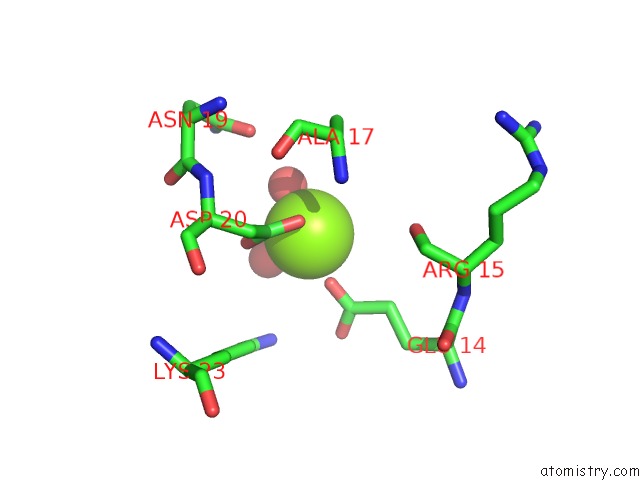
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 7 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
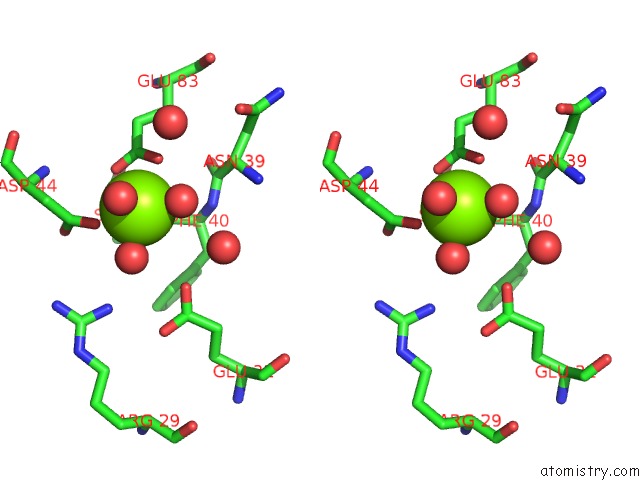
Magnesium binding site 8 out of 8 in 5f49
Go back to
Magnesium binding site 8 out
of 8 in the Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A
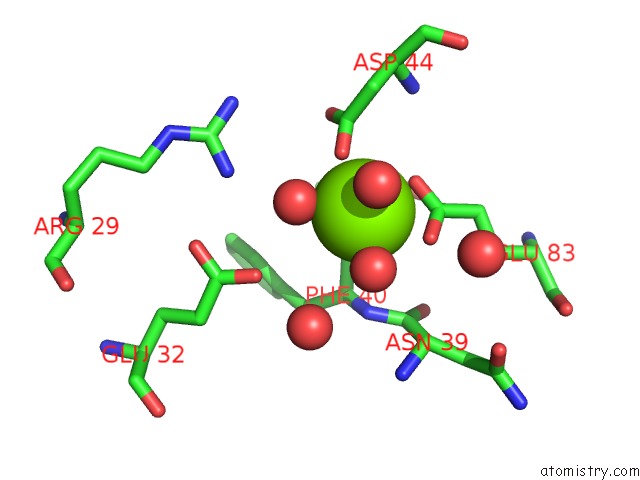
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 8 of Crystal Structure of An Aminoglycoside Acetyltransferase Meta-AAC0020 From An Uncultured Soil Metagenomic Sample in Complex with Malonyl- Coenzyme A within 5.0Å range:
|
Reference:
Z.Xu,
P.J.Stogios,
A.T.Quaile,
K.J.Forsberg,
S.Patel,
T.Skarina,
S.Houliston,
C.Arrowsmith,
G.Dantas,
A.Savchenko.
Structural and Functional Survey of Environmental Aminoglycoside Acetyltransferases Reveals Functionality of Resistance Enzymes. Acs Infect Dis V. 3 653 2017.
ISSN: ESSN 2373-8227
PubMed: 28756664
DOI: 10.1021/ACSINFECDIS.7B00068
Page generated: Tue Aug 12 07:58:00 2025
ISSN: ESSN 2373-8227
PubMed: 28756664
DOI: 10.1021/ACSINFECDIS.7B00068
Last articles
Mg in 6VZSMg in 6VZT
Mg in 6VZR
Mg in 6VZQ
Mg in 6VZ8
Mg in 6VZ9
Mg in 6VYD
Mg in 6VZ4
Mg in 6VWP
Mg in 6VWW