Magnesium »
PDB 8e1u-8ec1 »
8e7v »
Magnesium in PDB 8e7v: Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
Enzymatic activity of Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
All present enzymatic activity of Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles:
3.4.21.92;
3.4.21.92;
Magnesium Binding Sites:
The binding sites of Magnesium atom in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
(pdb code 8e7v). This binding sites where shown within
5.0 Angstroms radius around Magnesium atom.
In total 3 binding sites of Magnesium where determined in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles, PDB code: 8e7v:
Jump to Magnesium binding site number: 1; 2; 3;
In total 3 binding sites of Magnesium where determined in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles, PDB code: 8e7v:
Jump to Magnesium binding site number: 1; 2; 3;
Magnesium binding site 1 out of 3 in 8e7v
Go back to
Magnesium binding site 1 out
of 3 in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
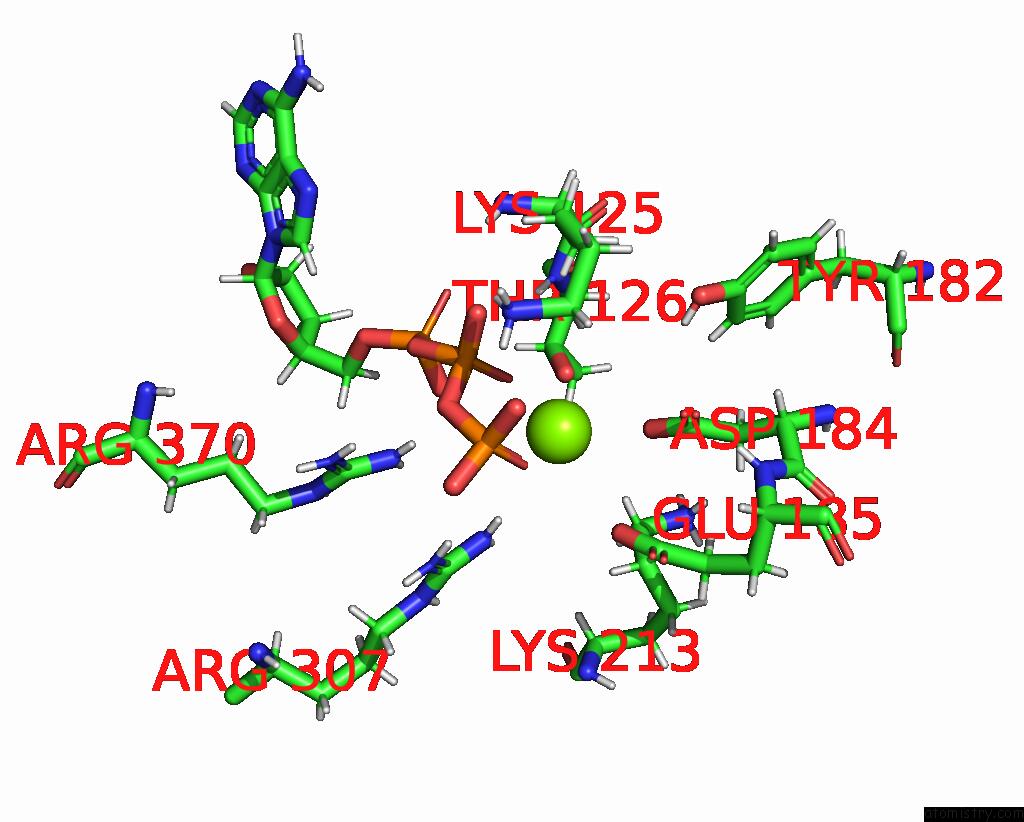
Mono view
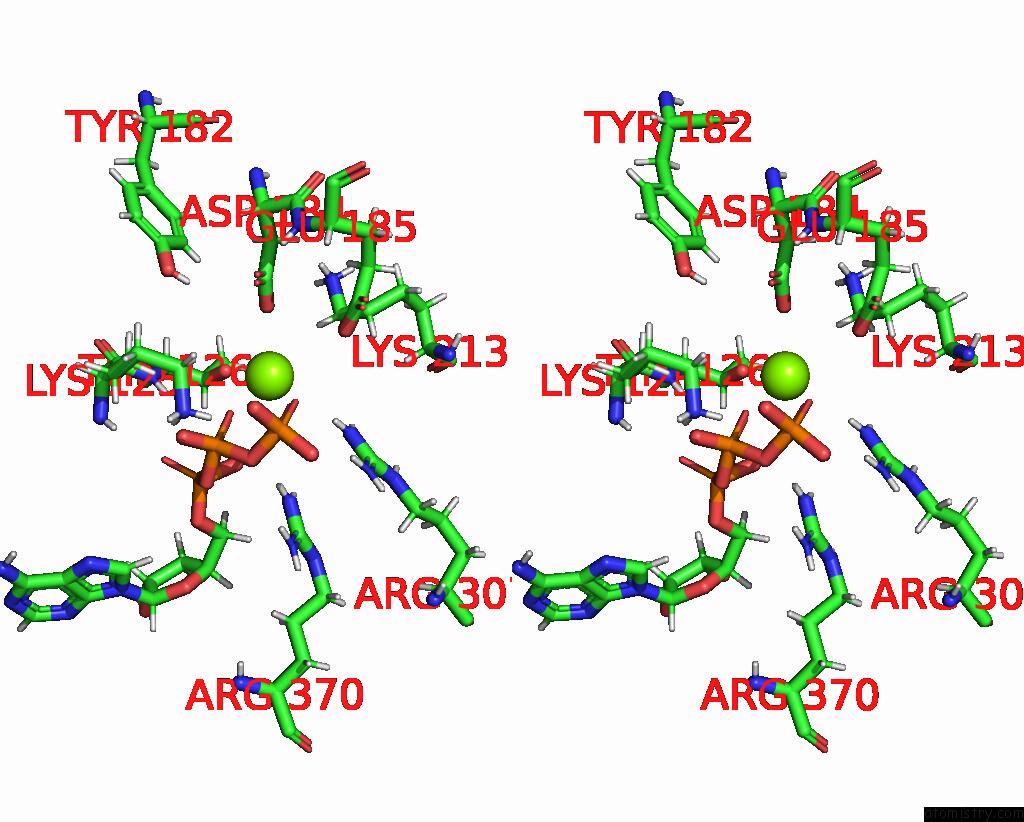
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 1 of Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles within 5.0Å range:
|
Magnesium binding site 2 out of 3 in 8e7v
Go back to
Magnesium binding site 2 out
of 3 in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
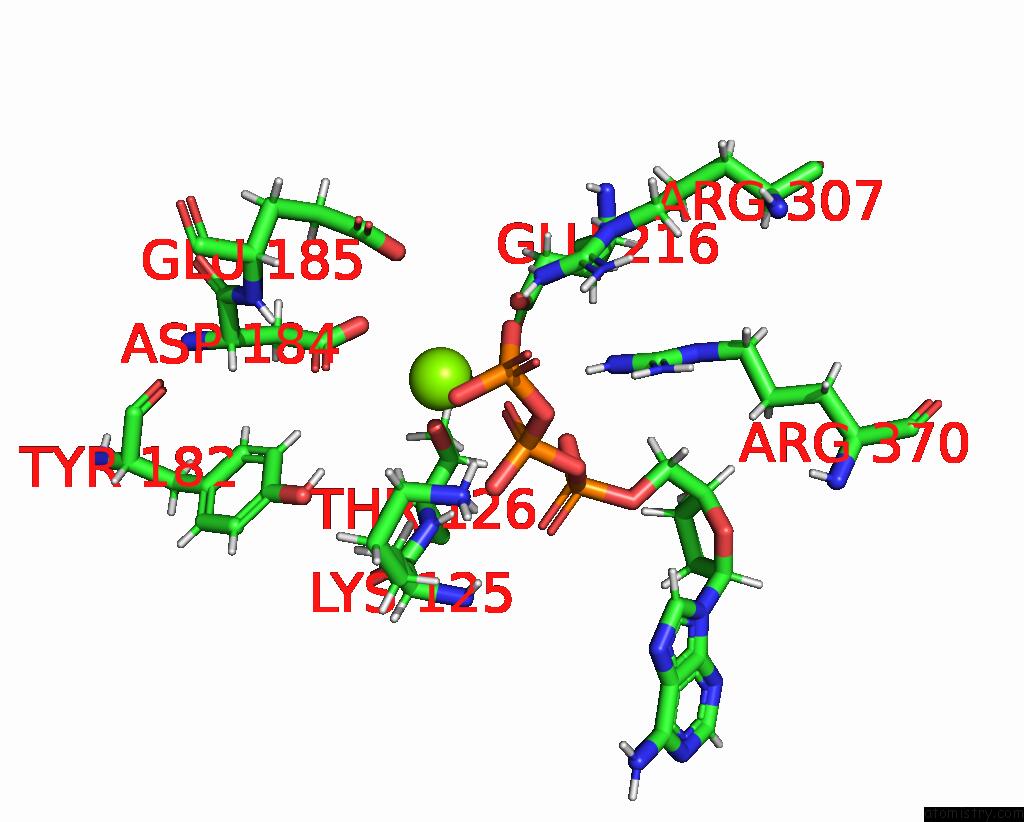
Mono view
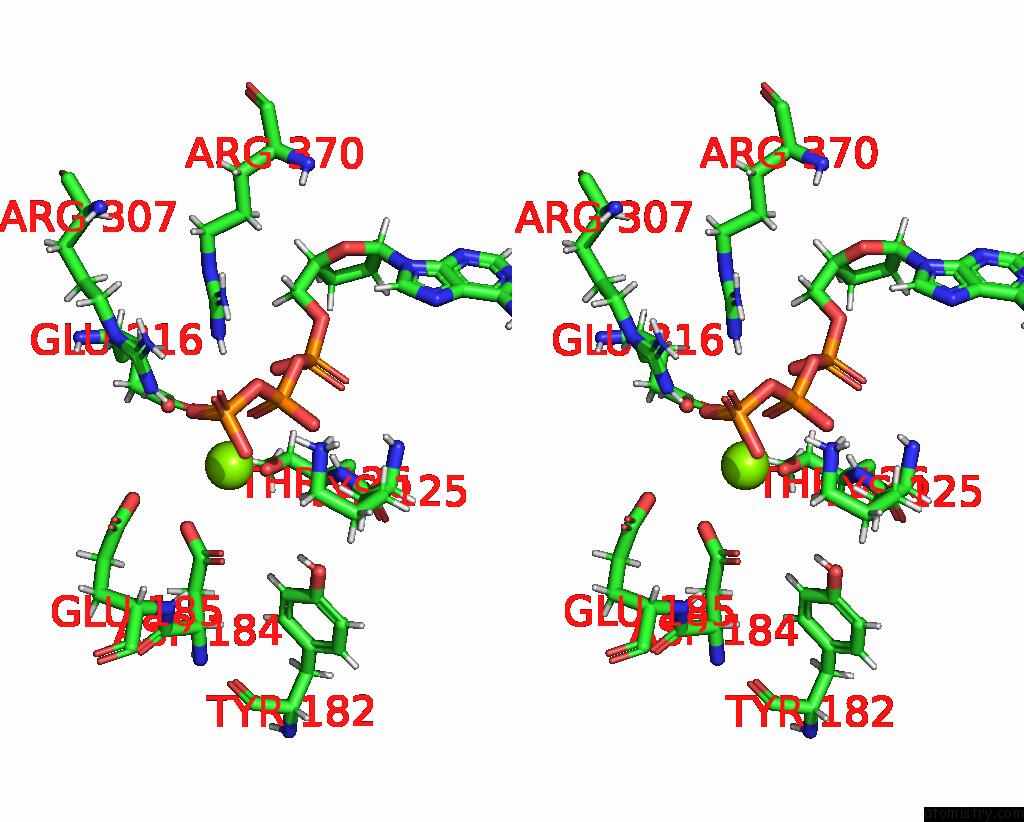
Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 2 of Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles within 5.0Å range:
|
Magnesium binding site 3 out of 3 in 8e7v
Go back to
Magnesium binding site 3 out
of 3 in the Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles
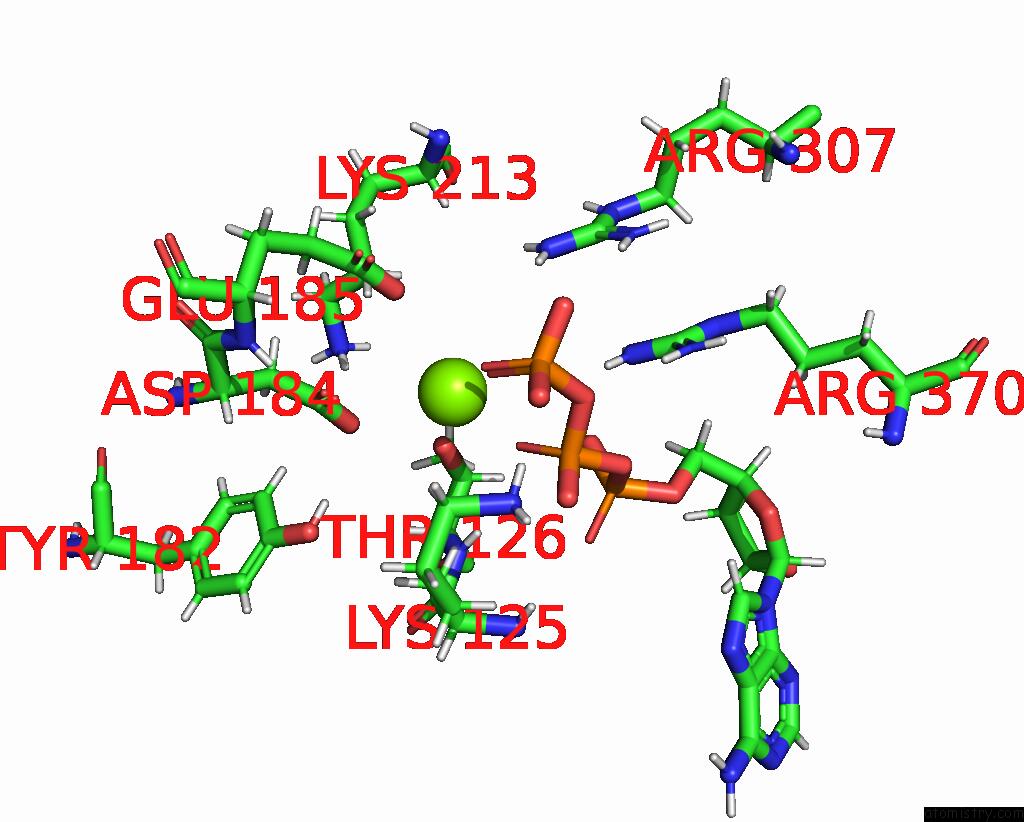
Mono view

Stereo pair view

Mono view

Stereo pair view
A full contact list of Magnesium with other atoms in the Mg binding
site number 3 of Cryo-Em Structure of Substrate-Free Dnclpx.Clpp From Singly Capped Particles within 5.0Å range:
|
Reference:
A.Ghanbarpour,
S.Cohen,
J.H.Davis,
R.T.Sauer.
Cryo-Em Structure of Substrate-Free Dnclpx.Clpp Nat Commun 2023.
ISSN: ESSN 2041-1723
Page generated: Fri Aug 15 03:39:26 2025
ISSN: ESSN 2041-1723
Last articles
Mg in 8H68Mg in 8H5Y
Mg in 8H4P
Mg in 8H5M
Mg in 8H4G
Mg in 8H4F
Mg in 8H4D
Mg in 8H4E
Mg in 8H4C
Mg in 8H4B